
May 16, 2020 Bromine (Z=35), which has 35 electrons, can be found in Period 4, Group VII of the periodic table. Since bromine has 7 valence electrons, the 4s orbital will be completely filled with 2 electrons, and the remaining five electrons will occupy the 4p orbital. Electrons have a specific form of distribution (or configuration) in every atom, even Bromine. Some are hard to memorise (or predict), so what is the electron configuration of an atom of Br? In the case of Bromine the abbreviated electron configuration is Ar 3d10 4s2 4p5. Nevertheless, check the complete configuration and other interesting.
- Chlorine, bromine and Iodine have empty n-‘d’ orbital. These elements when combining with the more electronegative element, their electrons of nth orbit get promoted to n-‘d’ orbital. Therefore, they can show positive oxidation states like +1, +3, +5 and +7.
- In the case of Bromine the abbreviated electron configuration is Ar 3d10 4s2 4p5. Nevertheless, check the complete configuration and other interesting facts about Bromine that most people don't know.
Click to see full answer
In respect to this, what happens when a bromine ion becomes an ion?
IONS Bromine Can MakeTo become an ion, an element has to gain or loose electrons. If it gains electrons, it receives a negative charge because it then has more electrons than protons. This is known as an anion. If it looses electrons, it receives a positive charge because it has more protons than electrons.
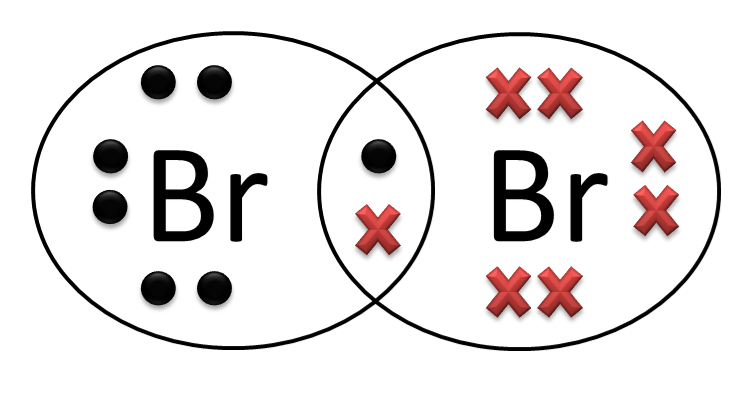
Also, does bromine gain or lose electrons? Bromine atoms tend to gain just one electron to get to a full octet, as Bromine is in Group VII. A chemical consisting of an aluminum ion and a bromide ion in their stable states would be AlBr2+, but it is not an ionic compound because it has a charge. Thus it tends to lose two electrons.
Similarly, will a bromine atom form a positive or negative ion Why?
The neutral atom of bromine has 35 electrons because the number of electrons equals the number of protons. c) Bromine gains an electron, what is the resulting ion called and is it positively or negatively charged? When bromine gains an electron, the resulting ion is called an anion and is negatively charged.
What type of ion does bromine form?
Bromine Electrons Gained Or Lost
A bromide is a chemical compound containing a bromide ion or ligand. This is a bromine atom with an ionic charge of −1 (Br−); for example, in caesium bromide, caesium cations (Cs+) are electrically attracted to bromide anions (Br−) to form the electrically neutral ionic compound CsBr.


